Evolution of Primate Dentition
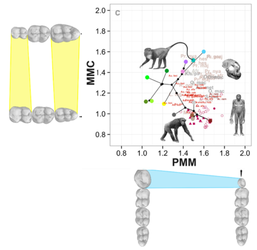
In collaboration with the Hlusko Lab at the Human Evolution Research Center at UC Berkeley, we are investigating novel genetically-patterned dental phenotypes that give far greater resolution to the evolutionary pathways of dentition in primates (and fossil hominins) than previously used metrics. These pathways are based on the genetically patterned size relationships within the molar row (MMC) and the developmental relationship between the premolars and molars (PMM).
See our 2016 paper in PNAS for more detail and information, including a discussion of our work and its implications for studies of evolution in a paleontological context by David Polly.
My current work on this project focuses on extending these metrics and methods to better understand dental evolution primate groups not previously investigated, including the platyrrhines (monkeys of the Americas).
See our 2016 paper in PNAS for more detail and information, including a discussion of our work and its implications for studies of evolution in a paleontological context by David Polly.
My current work on this project focuses on extending these metrics and methods to better understand dental evolution primate groups not previously investigated, including the platyrrhines (monkeys of the Americas).
Recent publications associated with this project include:
Monson et al. (2022, Biology (MDPI) 11: 1218) takes a long view towards understanding how the genetically patterned dental traits our group developed reinforce the importance of fossil data to understanding dental evolution among primates and hominins, in particular. We use ancestral state reconstruction to demonstrate that modeling using our genetically-patterned traits miss key phenotypic states that can only be tracked using fossil data, emphasizing that combination of gene-forward modeling and engagement with fossil data is needed to understand the evolutionary history of dental variation.
Brasil et al. (2020, Naturwissenschaften 107: 40) uses our genetically patterned dental traits to investigate variation in the hominin fossil record, and pushes forward the idea of using these traits to characterize evolutionary modes in the hominin fossil record that may inform hominin taxonomy.
Monson et al. (2019, Ecology & Evolution 9: 7597-7612) tracks variation in genetically-patterned dental traits through the entire Boreoeutheria, and in so doing discovered no association with dental variation and diet with tooth morphology changing much more slowly than dietary shifts, suggesting strong stabilizing selection in dental morphology.
Hlusko et al. (2016, PNAS 113: 9262-7) establishes our two genetically-patterned dental phenotypes, the molar module (MMC) and premolar-molar module (PMM), and assesses their ability to characterize evolutionary patterns in dentition across catarrhine primates. We find that these traits are highly heritable with little environmental noise, that they are distinguishable between genera and species (and place fossils well in evolutionary morphospace), and that they effectively trace evolutionary patterns within catarrhines, including showing clear evidence of variation that recapitulates the mid-Miocene shift from ape to cercopithecoid prominence in Africa and Eurasia.
Monson et al. (2022, Biology (MDPI) 11: 1218) takes a long view towards understanding how the genetically patterned dental traits our group developed reinforce the importance of fossil data to understanding dental evolution among primates and hominins, in particular. We use ancestral state reconstruction to demonstrate that modeling using our genetically-patterned traits miss key phenotypic states that can only be tracked using fossil data, emphasizing that combination of gene-forward modeling and engagement with fossil data is needed to understand the evolutionary history of dental variation.
Brasil et al. (2020, Naturwissenschaften 107: 40) uses our genetically patterned dental traits to investigate variation in the hominin fossil record, and pushes forward the idea of using these traits to characterize evolutionary modes in the hominin fossil record that may inform hominin taxonomy.
Monson et al. (2019, Ecology & Evolution 9: 7597-7612) tracks variation in genetically-patterned dental traits through the entire Boreoeutheria, and in so doing discovered no association with dental variation and diet with tooth morphology changing much more slowly than dietary shifts, suggesting strong stabilizing selection in dental morphology.
Hlusko et al. (2016, PNAS 113: 9262-7) establishes our two genetically-patterned dental phenotypes, the molar module (MMC) and premolar-molar module (PMM), and assesses their ability to characterize evolutionary patterns in dentition across catarrhine primates. We find that these traits are highly heritable with little environmental noise, that they are distinguishable between genera and species (and place fossils well in evolutionary morphospace), and that they effectively trace evolutionary patterns within catarrhines, including showing clear evidence of variation that recapitulates the mid-Miocene shift from ape to cercopithecoid prominence in Africa and Eurasia.
Recent presentations by lab members associated with this project have included:
Schmitt CA, Cooke SB, Brasil MF, Monson TA, Hlusko LJ. 2018. Genetically patterneddental phenotypes show evidence for diet-related evolutionary change in platyrrhine primates. Annual Meeting of the American Association of Physical Anthropologists.
Schmitt CA, Cooke SB, Brasil MF, Monson TA, Hlusko LJ. 2018. Genetically patterneddental phenotypes show evidence for diet-related evolutionary change in platyrrhine primates. Annual Meeting of the American Association of Physical Anthropologists.